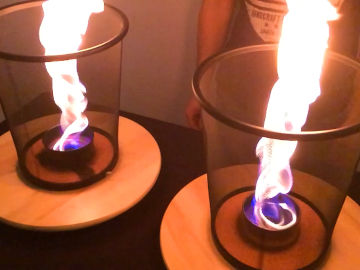Fun and easy science experiments for kids and adults.
Special:
Chemistry
Ignite alcohol inside an empty water dispenser bottle. Whoosh! An explosion followed by a pulsating blue fire. This is an experiment about combustion and states of matter.
| Gilla: | Dela: | |
Video

Materials
- 1 empty 18.9 L (5 gallon) water dispenser bottle
- Ethanol
- 1 matchbox (or lighter with a long neck, although it might give out due to the explosion)
- 1 jar with a lid
- Safety equipment: 1 fire extinguisher, 1 bucket of water, 1 pair of safety goggles
Warning!
These risks exist:- Something may catch fire.
- Someone may burn themselves.
- Inhalation, skin contact, eye contact or ingestion of ethanol.
- Do the demonstration in the company of an adult with experience of fire.
- Wear safety goggles.
- Have a fire extinguisher ready.
- Have a bucket of water ready.
- Never lean over the opening of the bottle.
- When the ethanol ignites and you withdraw your hand, be careful not to tip the bottle over - especially if you use a long-necked lighter.
- When you've finished the demonstration, rinse the can with water so that no ethanol is left.
- Practice what to do if something catches fire or if someone burn themselves.
- Practice what to do if someone is injured by ethanol:
- Inhalation: Rest. Move to fresh air. Get medical attention if necessary.
- Skin contact: Take off contaminated clothes and shoes. Wash off skin with plenty of water and soap. Get medical attention if necessary.
- Eye contact: Rinse immediately with plenty of water, also under the eyelids, for at least 15 minutes. Get medical attention if necessary.
- Ingestion: Rinse mouth. Drink plenty of water. Get medical attention.
Environment!
The small amounts of ethanol that might end up in the drain when rinsing the bottle is fine.Step 1


Step 2


Step 3


Step 4


Step 5


Short explanation
When you shake the bottle, the ethanol evaporates. This means that it changes from a liquid state to a gaseous state. Gaseous ethanol burns much faster than liquid ethanol, because it mixes better with oxygen.Long explanation
When you pour the ethanol into the bottle, some of the ethanol will change from a liquid state to a gaseous state (evaporate) and become invisible ethanol vapor in the bottle. A liquid always changes to gas to some extent, and vice versa, even if no change in temperature occurs. Evaporation takes place at the surface, i.e. where the liquid is in contact with the air. Therefore, shaking the bottle so it splashes speeds up evaporation. This is because lots of small drops of ethanol are created, which means more places where evaporation can take place, and evaporation thus takes place faster. There will also be air in the bottle. Air consists of 21 % oxygen. Ethanol and oxygen are two chemical substances that like to react with each other. In fact, it happens as soon as they come in contact with each other, albeit extremely slowly. But you can speed up the reaction by heating the substances, for example with the help of the heat from a match. The chemical reaction that occurs when ethanol burns is as follows: C2H5OH + 4 O2 → 2 CO2 + 3 H2O + energi A chemical reaction is when one or more chemical substances are formed from other chemical substances. The type of chemical reaction that occurs in this demonstration is called combustion. This is when one of the chemical substances that reacts is oxygen and energy is released. The chemical substance that reacts with oxygen is called a fuel. The energy released comes in the form of light (radiant energy) and heat (thermal energy). The flame in this demonstration consists of ethanol and oxygen that are being converted to water and carbon dioxide, as well as the radiant energy (light) and thermal energy (heat) that is formed as a by-product in that process. It's possible that part of the flame contains ethanol and/or oxygen that has become so hot that it's been ionized. This means that some of the electrons of these molecules are completely detached. This state is called plasma and is the state of matter that the chemical substances in the Sun have. But it's unclear whether the chemical reaction in this demonstration really releases so much heat that ionization occurs - and to our eyes, burning plasma and burning gas look pretty much the same. A good model for understanding fire is the so-called fire triangle. It says that three ingredients are required for combustion and fire: (1) heat, (2) fuel and (3) oxygen (or any other oxidant). A fire occurs when these three ingredients are present in the right mixture. A fire can also be extinguished by removing any of these three ingredients. The main point of this demonstration is to show that a gaseous fuel burns much more violently than a liquid or solid fuel. This is because all the molecules of the fuel have good contact with the oxygen molecules in the air. When, on the other hand, a fuel is in a liquid state, evaporation takes place only at the surface. It's only there the molecules of the fuel come into sufficiently good contact with the oxygen molecules in the air to be able to react with them. When a fuel is in a solid state, the situation is even worse. In addition, the fuel must first melt, and then evaporate. How the fire wanders down through the bottle will look different every time you do the demonstration. Sometimes it is super fast. Sometimes slow. Sometimes the fire comes and goes in short, fast pulses. It all depends on the mixture of ethanol and oxygen in the bottle. If the mixture is perfect, everything burns up quickly. If there is a deficiency of oxygen, it burns slowly. Maybe the combustion slows down almost completely, until new air is sucked into the bottle and it starts again, and you get a pulsating fire.Experiment
You can turn this demonstration into an experiment. This will make it a better science project. To do that, try answering one of the following questions. The answer to the question will be your hypothesis. Then test the hypothesis by doing the experiment.- What happens if you shake the bottle for 0, 10, 20 or 30 seconds respectively?
- What happens if you do not shake the bottle at all but just wait for 1 minute?
- What happens if you, after completing the demonstration once, wait for 10 minutes and then ignite whatever is in the bottle again?
- What happens if you, after completing the demonstration once, immediately try to ignite it again?
Variation
A cool variation is to put your hand over of the opening after the fire. However, make sure the fire is really out! Your hand will then be pressed firmly against the opening, the bottle will start to crumple and you can then lift the bottle - all accompanied by cool sound effects. This is because the gas mixture in the bottle cools and contracts.| Gilla: | Dela: | |
Similar
Latest
Content of website
© The Experiment Archive. Fun and easy science experiments for kids and adults. In biology, chemistry, physics, earth science, astronomy, technology, fire, air and water. To do in preschool, school, after school and at home. Also science fair projects and a teacher's guide.
To the top
© The Experiment Archive. Fun and easy science experiments for kids and adults. In biology, chemistry, physics, earth science, astronomy, technology, fire, air and water. To do in preschool, school, after school and at home. Also science fair projects and a teacher's guide.
To the top