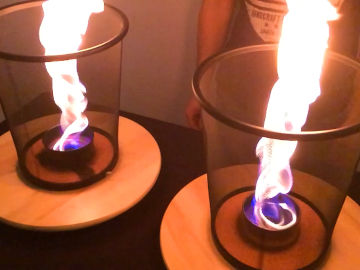Fun and easy science experiments for kids and adults.
Chemistry
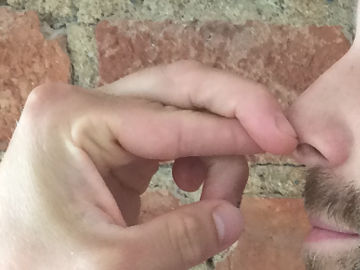
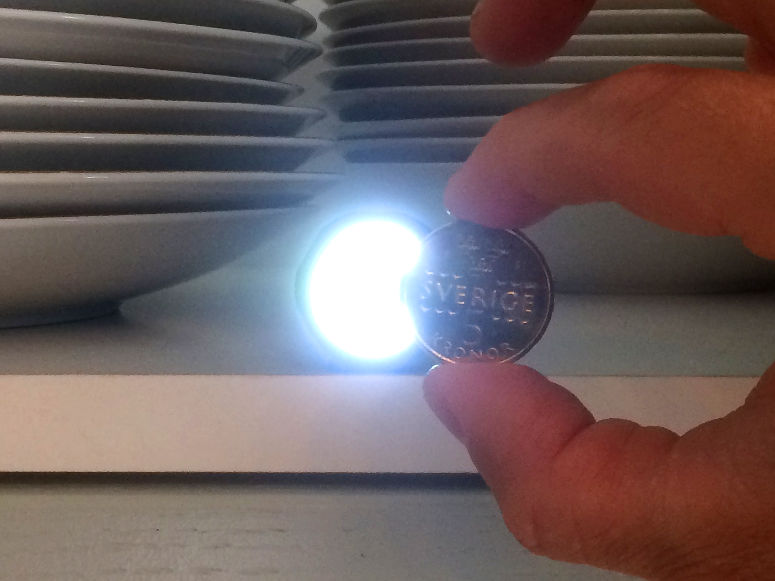
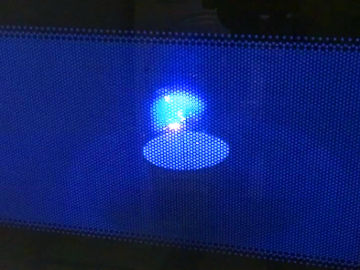
Relight a candle using the smoke. This is an experiment about the science of a burning candle.
| Gilla: | Dela: | |
Video

Materials
- 1 candle (the more smoke it emits when you blow it out, the better)
- 1 lighter or matchbox
- Safety equipment: 1 fire extinguisher
Warning!
Fire is present in this demonstration. A fire extinguisher must be close at hand.Step 1


Step 2


Step 3


Step 4


Short explanation
The smoke from a blown out candle is vaporized wax (or other constituents of the candle). When a candle burns, it is in fact this evaporated wax that burns, and you can easily light it on fire again. However, the fire spreads lightning fast down to the wick, where there is a good supply of new wax that evaporates.Long explanation
When lighting a candle, you use an open flame from a match or lighter that you bring to the wick. The wick, which during manufacture was dipped in some combustible substance such as wax, then begins to burn. The heat from the burning wick causes the candle's top layer of wax to melt. The molten wax travels up through the wick by capillary force, which means that the molecules of the wax, through electrical attraction, travel up along the walls in small passages in the wick. When the wax reaches a bit up the wick, the heat causes it to evaporate, i.e. to change from a liquid state to a gaseous state. After that, the heat causes the evaporated wax to start burning as well. The burning wax melts new wax in the top layer of the candle, which travels up through the wick and so on. The candle is thus self-sufficient after it has been lit. A wax candle usually consists of paraffin wax. But also a mixture of lots of other chemical substances. Common to all these chemical substances, however, is that their molecules contain many carbon and hydrogen atoms. When this fuel burns its molecules react with oxygen in the air in a chemical reaction. This only happens at a sufficiently high temperature. The carbon and hydrogen atoms in the fuel, as well as the oxygen atoms in the air, then form carbon dioxide (consists of carbon and oxygen atoms) and water (consists of hydrogen and oxygen atoms). Both carbon dioxide and water will be in a gaseous state and will not be visible. During the chemical reaction, some chemical energy is released in the form of light and heat. But what is the smoke from a candle? Well, there is usually no complete combustion of the fuel and some evaporated paraffin wax (and other constituents of the candle) will end up in the air. This becomes especially noticeable when the candle is blown out. Then the heat disappears from the flame, and the chemical reaction stops. The evaporated candle fuel then rises in the air without being burned. If you add heat to this evaporated fuel, you can make it start burning again, and that's exactly what you do in the demonstration. You can ignite the evaporated fuel quite far away from the wick, but the fire will quickly spread down to the candle again where there is plenty of fuel. Why does a candle go out when you blow on it? Well, then you blow the evaporated and burning fuel away from the candle. When this happens, there's no longer any heat at the wick that can evaporate the liquid fuel in it. The candle runs out of fuel and goes out.Experiment
You can turn this demonstration into an experiment. This will make it a better science project. To do that, try answering one of the following questions. The answer to the question will be your hypothesis. Then test the hypothesis by doing the experiment.- How far from the candle can you hold the lighter and still make the candle start burning again?
- What type of candle works best for this demonstration?
| Gilla: | Dela: | |
Similar
Latest
Content of website
© The Experiment Archive. Fun and easy science experiments for kids and adults. In biology, chemistry, physics, earth science, astronomy, technology, fire, air and water. To do in preschool, school, after school and at home. Also science fair projects and a teacher's guide.
To the top
© The Experiment Archive. Fun and easy science experiments for kids and adults. In biology, chemistry, physics, earth science, astronomy, technology, fire, air and water. To do in preschool, school, after school and at home. Also science fair projects and a teacher's guide.
To the top