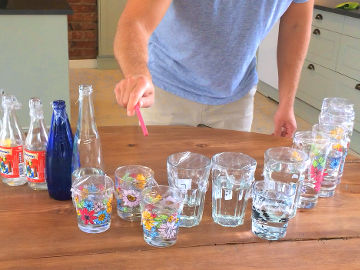Fun and easy science experiments for kids and adults.
Chemistry
Create a color explosion in milk using dish soap and food coloring. This is an experiment about polar and nonpolar substances, water and mixtures.
| Gilla: | Dela: | |
Video

Materials
- Milk with 3-4 % fat
- 1 plate
- Food coloring (the more colors the better)
- Liquid dish soap
- 1 cotton swab ("Q-tip")
Step 1

Step 2


Step 3


Step 4


Short explanation
When the dish soap is added to the milk, two things happen; the surface tension of the water decreases and tiny clumps of fat surrounded by dish soap form. Both of these things make the food coloring move.Long explanation
Milk is a mixture consisting of water and chemical substances dissolved in the water. Water consists of water molecules that attract each other quite strongly. You can see this when the food colors has difficulty mixing with the water and remains as drops in a limited area. But when dish soap is added to water, the dish soap molecules penetrate in between the water molecules and break their bonds. The food coloring molecules can then move freely and spread out in the milk. The dish soap also has another effect. Dish soap consist of molecules whose one end attaches to fat molecules in the milk and the other end to water molecules. The result is small aggregations of fat molecules surrounded by dish soap molecules. These small constellations, called micelles, are formed quite vigorously and push away other molecules in the formation process. The reason why water molecules attract each other is that they all have a positively charged end and a negatively charged end. Water molecules are polar. They can be compared to a bunch of magnets, which also have a positive and a negative end (these ends are magnetic instead of electrically charged, but the forces that arise work the same way). If these magnets were thrown in a bucket, they would line up, with positive ends against negative ends, and hold together. Other chemical substances whose molecules are also polar mix easily with water. It's more difficult for chemical substances that are non-polar, i.e. whose molecules have no charged ends. Food coloring, for example, is relatively non-polar, and therefore does not mix easily with water. Fat molecules are non-polar and therefore do not mix with water. This can be seen by the fact that fat in water is still held together like large drops. In milk, therefore, the fat is not very well mixed, but exists in these drops. Dish soap consist of molecules that have a polar and a non-polar end. These molecules can mix with both water and fat. When dish soap is mixed into milk, or another high-fat mixture, its molecules act as mediators. One end attaches to fat molecules and the other end to water molecules. The fat is now divided into much smaller droplets, which are called micelles, which are surrounded by dish soap molecules. Dish soap thus causes fat to partially dissolve in water.Experiment
You can turn this demonstration into an experiment. This will make it a better science project. To do that, try answering one of the following questions. The answer to the question will be your hypothesis. Then test the hypothesis by doing the experiment.- What happens if you use milk with another fat content?
- What happens if you use another kind of dairy product?
- What happens if you use another kind of dish soap?
- What happens if you use a cleaning agent instead of dish soap?
- What happens if you change the amount of dish soap?
| Gilla: | Dela: | |
Similar
Latest
Content of website
© The Experiment Archive. Fun and easy science experiments for kids and adults. In biology, chemistry, physics, earth science, astronomy, technology, fire, air and water. To do in preschool, school, after school and at home. Also science fair projects and a teacher's guide.
To the top
© The Experiment Archive. Fun and easy science experiments for kids and adults. In biology, chemistry, physics, earth science, astronomy, technology, fire, air and water. To do in preschool, school, after school and at home. Also science fair projects and a teacher's guide.
To the top