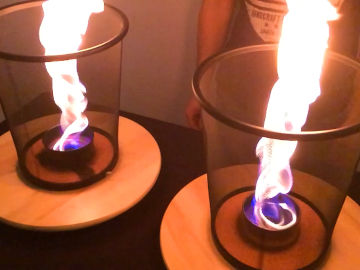Fun and easy science experiments for kids and adults.
Chemistry
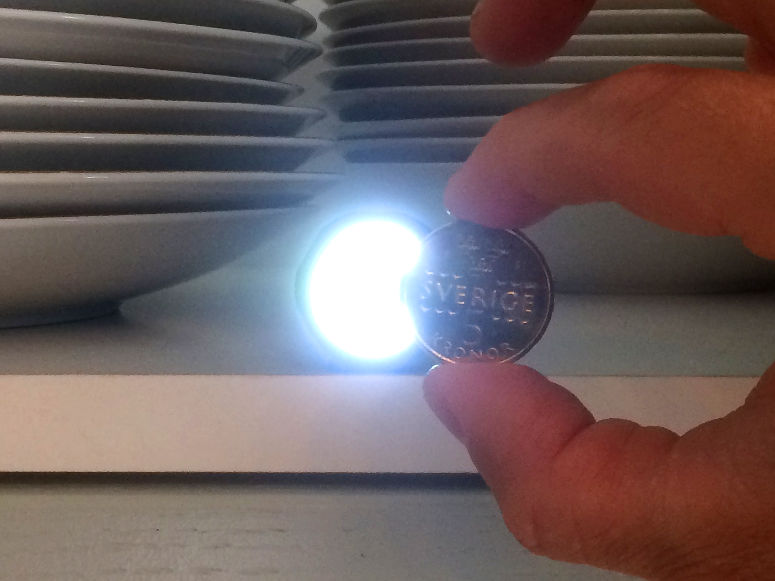
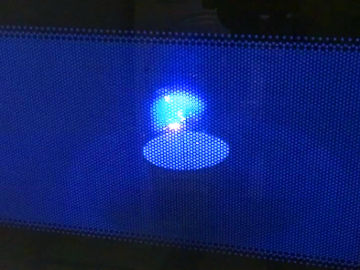
Make a candle from an orange and some cooking oil. This is an experiment about chemical reactions and states of matter.
| Gilla: | Dela: | |
Video

Materials
- 1 orange, clementine, satsuma or other citrus fruit
- 1 plate
- 1 knife
- 1 lighter
- Cooking oil
- Safety equipment: 1 fire extinguisher
Warning!
Fire is present in this demonstration. A fire extinguisher must be close at hand.Steg 1


Step 2


Step 3


Step 4


Step 5 (extra)


Short explanation
The thing that burns is the cooking oil. The heat causes the cooking oil in the wick to evaporate and then ignite. Then more cooking oil is sucked up into the wick.Long explanation
The orange candle works just like a normal candle, with the difference that the fuel is cooking oil instead of wax. When you light a candle you use a flame from a match or lighter that you bring to the wick. The heat from the flame makes the fuel the wick is soaked in (in this case cooking oil) to first change to a gaseous state in the vicinity of the wick, and then to burn. When this happens, you can remove the match or lighter, because the candle now has its own flame. As the fuel in the wick is being spent, new fuel moves up into the wick. This is done by capillary action, which means that the fuel molecules move up along the walls of small passages in the wick thanks to electrical attraction. In order for the fuel to be able to move up in the wick in this way, it must be liquid. The cooking oil in this demonstration is already liquid. In a wax candle the wax gets liquid when it melts from the heat of the flame. When the liquid fuel reaches some distance up the wick, the heat causes it to evaporate, i.e. to change from a liquid state to a gaseous state. After that, the heat also causes the fuel to start burning. And so it continues - the candle is self-sufficient. Cooking oil can be of different varieties and consist of slightly different types of molecules. What these molecules all have in common is that they contain lots of carbon and hydrogen atoms. The burning of cooking oil means that these molecules react with oxygen in the air in a chemical reaction. This only happens at a sufficiently high temperature. The carbon and hydrogen atoms in the fuel as well as the oxygen atoms in the air then form carbon dioxide (consists of carbon and oxygen atoms) and water (consists of hydrogen and oxygen atoms). Both carbon dioxide and water will be in a gaseous state and will not be visible. During the chemical reaction, some chemical energy in the cooking oil is released in the form of light and heat. Why does a candle go out when you blow it? Well, then you blow the evaporated and burning fuel away from the candle. When this happens, there is no longer any heat at the wick that can evaporate the liquid fuel in it. The candle runs out of fuel and goes out.Experiment
You can turn this demonstration into an experiment. This will make it a better science project. To do that, try answering one of the following questions. The answer to the question will be your hypothesis. Then test the hypothesis by doing the experiment.- What fruit makes the best candle?
- What kind of cooking oil makes the best fuel?
- For a long can 1 spoonful, 2 spoonfuls etc. of cooking oil sustain the candle?
| Gilla: | Dela: | |
Similar
Latest
Content of website
© The Experiment Archive. Fun and easy science experiments for kids and adults. In biology, chemistry, physics, earth science, astronomy, technology, fire, air and water. To do in preschool, school, after school and at home. Also science fair projects and a teacher's guide.
To the top
© The Experiment Archive. Fun and easy science experiments for kids and adults. In biology, chemistry, physics, earth science, astronomy, technology, fire, air and water. To do in preschool, school, after school and at home. Also science fair projects and a teacher's guide.
To the top