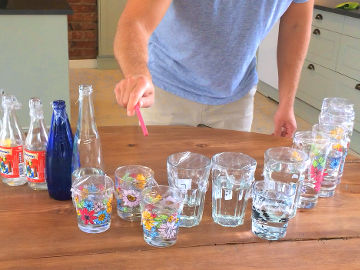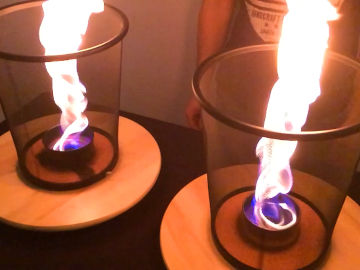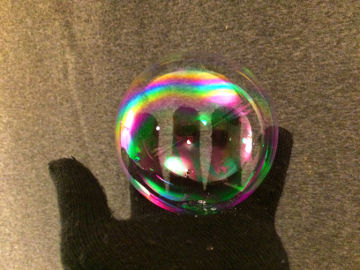Fun and easy science experiments for kids and adults.
Special:
Physics
Hold fire in your hand! This is an experiment about energy, heat, pressure, states of matter, chemical reactions and water.
| Gilla: | Dela: | |
Video

Materials
- 1 air freshener spray
- 1 large bowl
- Liquid dish soap
- 1 lighter or burning candle (for igniting the bubbles)
- Water
- Safety equipment: 1 fire extinguisher, 1 bucket of water, 1 pair of safety goggles
Warning!
These risks exist:- Something may catch fire.
- Someone may burn themselves.
- Do the demonstration in the company of an adult with experience of fire.
- Wear safety goggles.
- Have a fire extinguisher ready.
- Have a bucket of water ready.
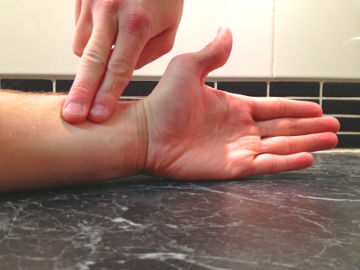
- The arm you will use must be thoroughly soaked with water.
- Hold your bubbles as far away from your face as you can. Also keep your hand above hair height and with the palm facing up. Make your palm flat.
- If the ceiling is low - sit on a chair.
- Do not do the demonstration outdoors, as the slightest wind can cause the flame to reach your face.
- Practice what to do if something catches fire or if someone burn themselves.
Step 1


Step 2


Step 3


Step 4


Step 5


Step 6


Step 7


Short explanation
In the bubbles you have trapped flammable gas from the spray bottle. When you move the lighter towards the bubbles, the gas heats up and it starts to burn. You do not burn yourself because the water in the bubbles and on your hand absorbs the heat instead.Long explanation
The aerosol spray bottle is an ingenious invention from the 1920s by the Norwegian Erik Rotheim. In a spray bottle there are usually two liquids mixed with each other. One liquid is the product, i.e. what you want when you buy the spray bottle. It can be, for example, insecticide, paint, deodorant, shaving foam or an air freshener liquid. The second liquid is for expelling the product from the spray bottle. This liquid is called the propellant. The propellant is actually a gas at room temperature. But during production, so much of the propellant gas has been pumped into the bottle that it has become liquid. A gas becomes liquid if the pressure becomes high enough (or if the temperature becomes low enough). When you press the nozzle of the spray bottle, a valve opens and both the propellant and the product come out. In the nozzle, the product is atomized into small droplets. At the same time, the propellant that comes out turns into a gas (it boils), due to the now low pressure. In shaving foam, among other things, the propellant gas remains as bubbles in the product and turns it into foam, but once there it has no more function. You might think that the pressure in the spray bottle decreases with each spray, but this isn't the case. This is because some of the liquid propellant in the bottle constantly changes to gas and resets the pressure. When you start using the bottle, it thus contains not only a liquid mixture of the product and the propellant, but also a layer of propellant in a gaseous state on top of this mixture. The propellant used in air fresheners usually consists of one or a few alkanes. The air freshener in the photos contained 50 % butane and 50 % propane, and we will use that content as an example here. All alkanes react at a sufficiently high temperature with oxygen, i.e. it burns. Therefore, there is a warning symbol for "flammable" on the spray bottle. When you spray with the spray bottle in the soap water, bubbles form. These bubbles are ordinary soap bubbles consisting of a layer of water surrounded by a layer of dish soap on each side. But the soap bubbles in this demonstration do not contain air, but the alkanes butane and propane (as well as small drops of lemon liquid). The alkanes are transparent just like air but, unlike air, they burn easily. When you move the lighter towards the soap bubbles, the gas in them heats up so much that it starts to burn. What happens then is that butane and propane react rapidly with oxygen and form water and carbon dioxide. This chemical reaction is exothermic, which means that energy is released. In this chemical reaction, energy is released in the form of radiant energy (including light) and thermal energy (heat). Since energy can neither be created nor destroyed, only transformed, one may ask where this energy came from? Well, when the butane, propane, and oxygen molecules were formed (whenever that was), energy was required to move the electrons in those molecules so that the constituent atoms would stay together. This energy was then stored as potential energy in these molecules, which is called chemical energy. That is, until now, when the electrons in this chemical reaction were moved to less energy-demanding positions in newly formed molecules. The flame in this demonstration consists of propane, butane and oxygen that are being converted to water and carbon dioxide, as well as the radiant energy (light) and thermal energy (heat) that is formed as a by-product in that process. It is possible that part of the flame contains propane, butane and/or oxygen that has become so hot that it has been ionized. This means that some of the electrons of these molecules are completely detached. This state is called plasma and is the state of matter that the substances in the Sun have. But it is unclear whether the chemical reaction in this demonstration really releases so much heat that ionization occurs - and to our eyes, burning plasma and burning gas look pretty much the same. The big question, though, is: why don't you burn your hand in this demonstration? The answer is water. The water on your hand and the water in the soap bubbles absorbs most of the energy released in the exothermic chemical reaction. Temperature is a measure of how much the particles in a substance move. In cold water the water molecules move a little and in hot water they move a lot. But it takes a lot of energy to heat water and get the molecules moving. This is because much of the energy added to water is used to bend and break the hydrogen bonds that hold the individual water molecules together. A lot of energy is absorbed by this process, instead of causing the water molecules to start moving. So water can be heated and heated, but still remain quite cool. Another way to put it is that water has a high heat capacity.Variation


This demonstration is also known as "methane mamba", but this is when you use methane instead of an aerosol spray. Methane must be bought from special stores and requires special safety measures. The result is pretty much the same.
| Gilla: | Dela: | |
Similar
Latest
Content of website
© The Experiment Archive. Fun and easy science experiments for kids and adults. In biology, chemistry, physics, earth science, astronomy, technology, fire, air and water. To do in preschool, school, after school and at home. Also science fair projects and a teacher's guide.
To the top
© The Experiment Archive. Fun and easy science experiments for kids and adults. In biology, chemistry, physics, earth science, astronomy, technology, fire, air and water. To do in preschool, school, after school and at home. Also science fair projects and a teacher's guide.
To the top