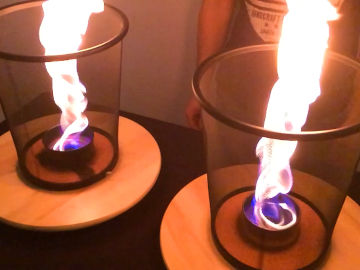Fun and easy science experiments for kids and adults.
Special:
Chemistry
Create a cloud of burning flour. This is an experiment about chemical reactions and fire.
| Gilla: | Dela: | |
Video

Materials
- 1 candle in a candlestick
- 1 lighter or matchbox
- 1 funnel
- 1 hose, at least 50 cm (20 in) long and which fits over the funnel spout
- Wheat flour (alternatively corn starch, potato flour, or powdered sugar)
- 1 spoon
- Safety equipment: 1 fire extinguisher, 1 bucket of water, 1 pair of safety goggles
Warning!
These risks exist:- Something may catch fire.
- Someone may burn themselves.
- Do the demonstration in the company of an adult with experience of fire.
- Wear satefy goggles.
- Have a fire extinguisher ready.
- Have a bucket of water ready.
- Make sure you have plenty of space in the direction you are blowing and that nothing that can catch fire is nearby.
- Keep the funnel as far away from your face as you can. Also keep the funnel above head height, which means you need to sit down.
- Do not do the demonstration outdoors, as the slightest wind can cause the flame to reach your face.
- Practice what to do if something catches fire or if someone burn themselves.
Step 1


Step 2


Step 3


Step 4


Step 5


Short explanation
When you blow flour, it mixes with oxygen in the air. When this mixture is heated by the frame of the candle, you now have all three ingredients for fire: fuel, oxygen and heat.Long explanation
The main ingredient in wheat flour is starch. Starch is really long molecules composed of the smaller glucose molecule (C6H12O6). When the cloud of wheat flour is heated by the candle, it begins to burn. What happens is that starch rapidly reacts with oxygen and forms water and carbon dioxide. This is the chemical reaction: 6 O2 + C6H12O6 → 6 CO2 + 6 H2O + energy This chemical reaction is exothermic, which means that energy is released into the environment. In this specific chemical reaction, energy is released in the form of radiant energy (electromagnetic waves, including visible light) and thermal energy (kinetic energy of particles, often referred to as "heat"). Since energy never can be created or destroyed, only converted, one may ask where this energy came from? Well, when the glucose (which starch consists of) was formed in wheat, energy was required to move the electrons in these molecules so that the constituent atoms would sit together. This energy came from the Sun and was captured by the plant through photosynthesis. This energy was then stored as chemical energy in glucose, which the plant than converted to starch - that is, until now, when the electrons in this chemical reaction were moved to lower energy-demanding positions in new molecules. The flame in this demonstration is big. It consists of starch and oxygen that are being converted to water and carbon dioxide. What we see is the radiant energy in the form of light that is formed in that process. It's possible that part of the flame contains starch or oxygen that has become so hot that it has been ionized. This means that some of the electrons of these molecules are completely detached. This state is called plasma and is the state of matter that the chemical substances in the Sun have. But it's unclear whether the chemical reaction in this demonstration really releases so much heat that ionization occurs - and to our eyes, burning plasma and burning gas look pretty much the same. Almost all organic molecules - i.e. molecules that originate from living organisms - contain a lot of energy and burn easily, not just starch. Therefore, several foods work for this demonstration. But what they must have in common is that they are powders, and can create a cloud of dust. Because it's only when the fuel mixes well with the oxygen in the air that it can burn. This is why your flour packages at home have never self-ignited. A good model for understanding fire is the so-called fire triangle. It says that three ingredients are required for combustion and fire: (1) heat, (2) fuel and (3) oxygen (or any other oxidant). A fire occurs when these three ingredients are present in the right mixture. A fire can also be extinguished by removing any of these three ingredients. The phenomenon in this demonstration is called a dust explosion, something that has caused many tragic fatal accidents throughout history. They are a real risk in coal mines and mills - or wherever flammable dust is formed.Experiment
You can turn this demonstration into an experiment. This will make it a better science project. To do that, try answering one of the following questions. The answer to the question will be your hypothesis. Then test the hypothesis by doing the experiment.- What happens if you replace the wheat flour with potato flour?
- What happens if you replace the wheat flour with corn starch?
- What happens if you replace the wheat flour with powdered milk?
- What happens if you replace the wheat flour with powdered sugar?
- What happens if you replace the wheat flour with granulated sugar?
- What happens if you increase the distance between the funnel and the candle?
- What happens if you switch to a larger funnel?
Variations
You can build a more permanent device for making dust explosions. Use a nail and hammer to punch a hole in the lowest part of the side of a large, empty, paint can. Insert one end of a hose through the hole. Place an outdoor candle in the middle of the jar and then pour wheat flour around it. Place the jar on something fireproof and heat-resistant and make sure you have nothing above the paint can. It's also appropriate with some type of screens, because the flame can get big! Except that, take the same safety precautions as above. Then blow into the hose to create a cloud of flour around the candle. You can also put a lid on the jar, which flies away like a rocket during the dust explosion. A common fuel to use for dust explosions in schools is lycopodium powder, which is spores from clubmoss plants.| Gilla: | Dela: | |
Similar
Latest
Content of website
© The Experiment Archive. Fun and easy science experiments for kids and adults. In biology, chemistry, physics, earth science, astronomy, technology, fire, air and water. To do in preschool, school, after school and at home. Also science fair projects and a teacher's guide.
To the top
© The Experiment Archive. Fun and easy science experiments for kids and adults. In biology, chemistry, physics, earth science, astronomy, technology, fire, air and water. To do in preschool, school, after school and at home. Also science fair projects and a teacher's guide.
To the top