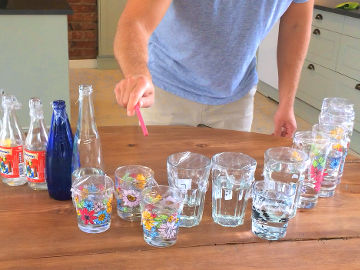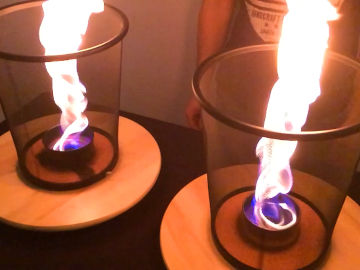Fun and easy science experiments for kids and adults.
Chemistry
Can you pour gas? In this experiment, a chemical reaction leads to an invisible gas with which you can extinguish candles.
| Gilla: | Dela: | |
Video

Materials
- 1 candle
- 1 matchbox or lighter
- 1 pitcher
- Baking powder
- Water
- Safety equipment: 1 fire extinguisher
Warning!
Fire is present in this demonstration. A fire extinguisher must be close at hand.Step 1


Step 2


Step 3


Step 4


Short explanation
When baking powder and water are mixed, carbon dioxide is formed. This gas is heavier than air and can be poured from a container. When you pour it over a burning candle, the flame goes out, because the oxygen needed for it to burn is pushed out of the way.Long explanation
When baking powder and water come into contact with each other, a chemical reaction takes place where carbon dioxide is formed. Carbon dioxide can then be poured over the flame that goes out. Baking powder consists of about 30 % baking soda, 40 % some acid (for example cream of tartar) and 30 % some moisture absorbing substance (for example corn starch). When baking powder and water are mixed, the baking powder begins to react with itself - bicarbonate and the baking powder's own acid reacts and forms a salt (which one depends on the acid) as well as carbon dioxide. Carbon dioxide in a gaseous state is heavier than air and therefore remains in the pitcher. The carbon dioxide isn't visible because it doesn't absorb or reflect light, but it is there. You can then pour the gas just like a liquid by tilting the pitcher. It's easier to hit the candle if there is as little turbulence in the air as possible, so an advice is to be very still around the candle.You can detect where the carbon dioxide is by lowering a lit match into the pitcher. It will be extinguished in the carbon dioxide, because there is no oxygen there. When you pour the carbon dioxide, it pushes away the lighter air. Because a candle needs the oxygen in the air to burn, it goes out.
This demonstration works in the same way as a carbon dioxide fire extinguisher does. It also releases carbon dioxide in order to displace air.
Experiment
You can turn this demonstration into an experiment. This will make it a better science project. To do that, try answering one of the following questions. The answer to the question will be your hypothesis. Then test the hypothesis by doing the experiment.- How many "intermediate pours", i.e. pouring from one glass to another, can you do and still have enough carbon dioxide left to extinguish the candle?
- How many candles at the most can you extinguish with a fixed amount of baking powder (i.e. how can you optimize all other factors)?
Variations
You can pour the carbon dioxide from one glass to another and then pour it over the candle. You can also use baking soda and vinegar instead of baking powder and water. Baking soda is also known as bicarbonate of soda. Both are household names for sodium bicarbonate. Common household vinegar, also called distilled white vinegar, consists of about 5 % acetic acid and the rest is water. When sodium bicarbonate and acetic acid mix they react and form sodium acetate, water and carbon dioxide.| Gilla: | Dela: | |
Similar
Latest
Content of website
© The Experiment Archive. Fun and easy science experiments for kids and adults. In biology, chemistry, physics, earth science, astronomy, technology, fire, air and water. To do in preschool, school, after school and at home. Also science fair projects and a teacher's guide.
To the top
© The Experiment Archive. Fun and easy science experiments for kids and adults. In biology, chemistry, physics, earth science, astronomy, technology, fire, air and water. To do in preschool, school, after school and at home. Also science fair projects and a teacher's guide.
To the top